Description
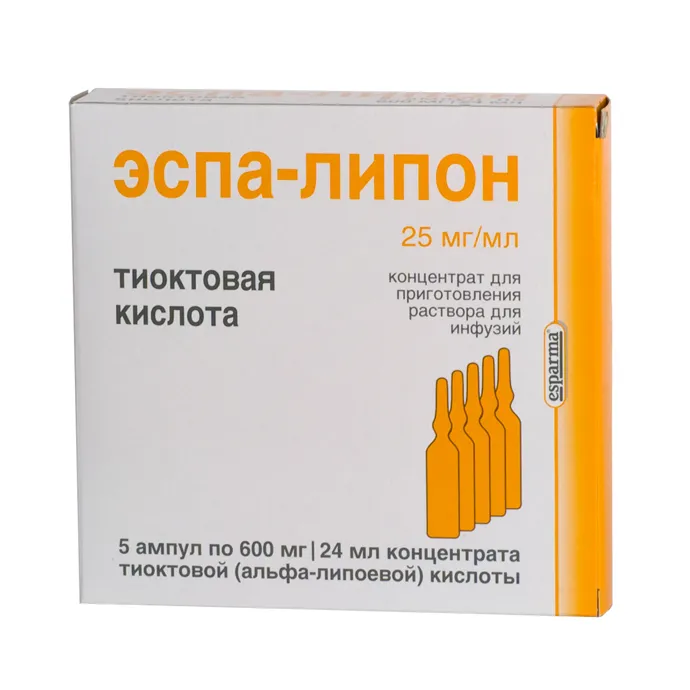
Espa-lipon (Thioctic Acid) Ampoules 600 mg. 24 ml. №5
Ingredients:
Each ampoule contains 600 mg of thioctic acid.
Mechanism of Action:
Thioctic acid, also known as alpha-lipoic acid, acts as a cofactor for mitochondrial enzymes involved in energy production. It exhibits antioxidant properties and is involved in managing conditions associated with oxidative stress.
Pharmacological Properties:
Thioctic acid supplementation has been shown to improve insulin sensitivity, reduce oxidative stress, and regenerate other antioxidants like vitamins C and E. It plays a role in improving nerve function and reducing neuropathic symptoms.
Indications for Use:
Espa-lipon ampoules are indicated for the treatment of diabetic neuropathy and other conditions associated with oxidative stress.
Contraindications:
Do not use Espa-lipon if allergic to thioctic acid or any other ingredients in the product. Consult a healthcare provider before use to assess individual suitability.
Side Effects:
Common side effects may include gastrointestinal discomfort. Rare but serious side effects may include allergic reactions. Consult a healthcare provider for a complete list of possible side effects.
Usage Instructions:
Administer the ampoules intravenously as directed by a healthcare professional. The recommended dosage should not be exceeded.
Benefits Compared to Analogues:
Espa-lipon offers the benefit of thioctic acid, a well-studied compound with antioxidant and neuroprotective properties, compared to other analogues. Its efficacy in managing diabetic neuropathy and oxidative stress has been supported by clinical evidence.
Suitable Patient Groups:
Espa-lipon can be used in various patient groups including adults, elderly individuals, and diabetic patients under healthcare provider supervision. Consult a healthcare provider for specific recommendations based on individual health conditions.
Storage Conditions and Shelf Life:
Store Espa-lipon in a cool, dry place away from direct sunlight. Check the expiration date on the packaging and do not use expired products.
Packaging Description:
Espa-lipon is available in ampoules containing 600 mg of thioctic acid in a 24 ml solution. Each package contains 5 ampoules for single-use intravenous administration.
Clinical Evidence and Proven Effectiveness:
Thioctic acid supplementation has been extensively studied for its role in managing diabetic neuropathy. Research published in the *International Journal of Endocrinology* has demonstrated its ability to improve neuropathic symptoms and nerve conduction velocity in diabetic patients.