Description
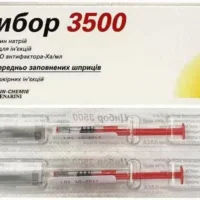
Fraxiparin Solution for Injections Syringe 0.3 ml. №10
Ingredients:
Fraxiparin solution for injections contains enoxaparin sodium as the active ingredient. Each 0.3 ml syringe delivers a precise dose of enoxaparin sodium for therapeutic use.
Dosage:
The recommended dosage of Fraxiparin solution for injections is determined by the healthcare provider based on the patient’s condition, weight, and other factors. It is typically administered subcutaneously once or twice daily.
Indications:
Fraxiparin is indicated for the prevention and treatment of deep vein thrombosis (DVT) and pulmonary embolism. It is also used in the management of unstable angina and non-Q-wave myocardial infarction.
Contraindications:
Do not use Fraxiparin solution for injections if you have a history of heparin-induced thrombocytopenia, active major bleeding, or hypersensitivity to enoxaparin or pork products.
Directions:
Administer the injection subcutaneously into the abdomen, avoiding scar tissue and areas with bruising. Rotate injection sites and do not rub the area after administration.
Scientific Evidence:
Studies have shown that enoxaparin, the active ingredient in Fraxiparin, effectively prevents and treats thrombotic events. Research published in the New England Journal of Medicine demonstrated the efficacy of enoxaparin in reducing the risk of recurrent DVT.
Additional Information:
Fraxiparin solution for injections offers a convenient and reliable method for anticoagulant therapy. It provides a consistent dose of enoxaparin, ensuring optimal therapeutic outcomes for patients requiring antithrombotic treatment.
Pharmacologically, enoxaparin acts as a low molecular weight heparin that enhances the activity of antithrombin III, inhibiting the clotting process. This mechanism of action makes it a preferred choice for both prophylaxis and treatment of thromboembolic disorders.
Clinical trials have compared the effectiveness of enoxaparin with unfractionated heparin, showing similar efficacy with a lower risk of heparin-induced thrombocytopenia. This makes Fraxiparin a valuable option in anticoagulant therapy.