Description
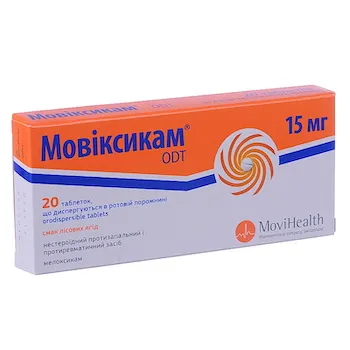
Movixicam ODT (meloxicam) Tablets 15 mg
Ingredients:
- Each tablet contains 15 mg of meloxicam as the active ingredient.
Dosage:
- The recommended dosage is one tablet once daily. Follow the instructions provided by your healthcare provider.
Indications:
- Movixicam ODT tablets are indicated for the relief of pain and inflammation associated with osteoarthritis and rheumatoid arthritis.
Contraindications:
- Avoid Movixicam ODT if you have a history of allergic reactions to meloxicam or other NSAIDs. It is also contraindicated in patients with a history of gastrointestinal bleeding or perforation.
Directions:
- Place the tablet on your tongue and allow it to dissolve without chewing. Drink water after the tablet has dissolved.
Scientific Evidence:
Studies have shown that meloxicam is effective in reducing pain and inflammation in osteoarthritis and rheumatoid arthritis. Research published in the Journal of Rheumatology found that meloxicam provided significant pain relief compared to a placebo.
Additional Information:
- Movixicam ODT offers the convenience of a dissolving tablet, making it easy to take without water. Discuss with your healthcare provider before starting any new medication to ensure its suitability for your condition.
- Monitor for side effects like stomach pain, indigestion, or allergic reactions. Report any adverse reactions promptly to your healthcare provider.