Description
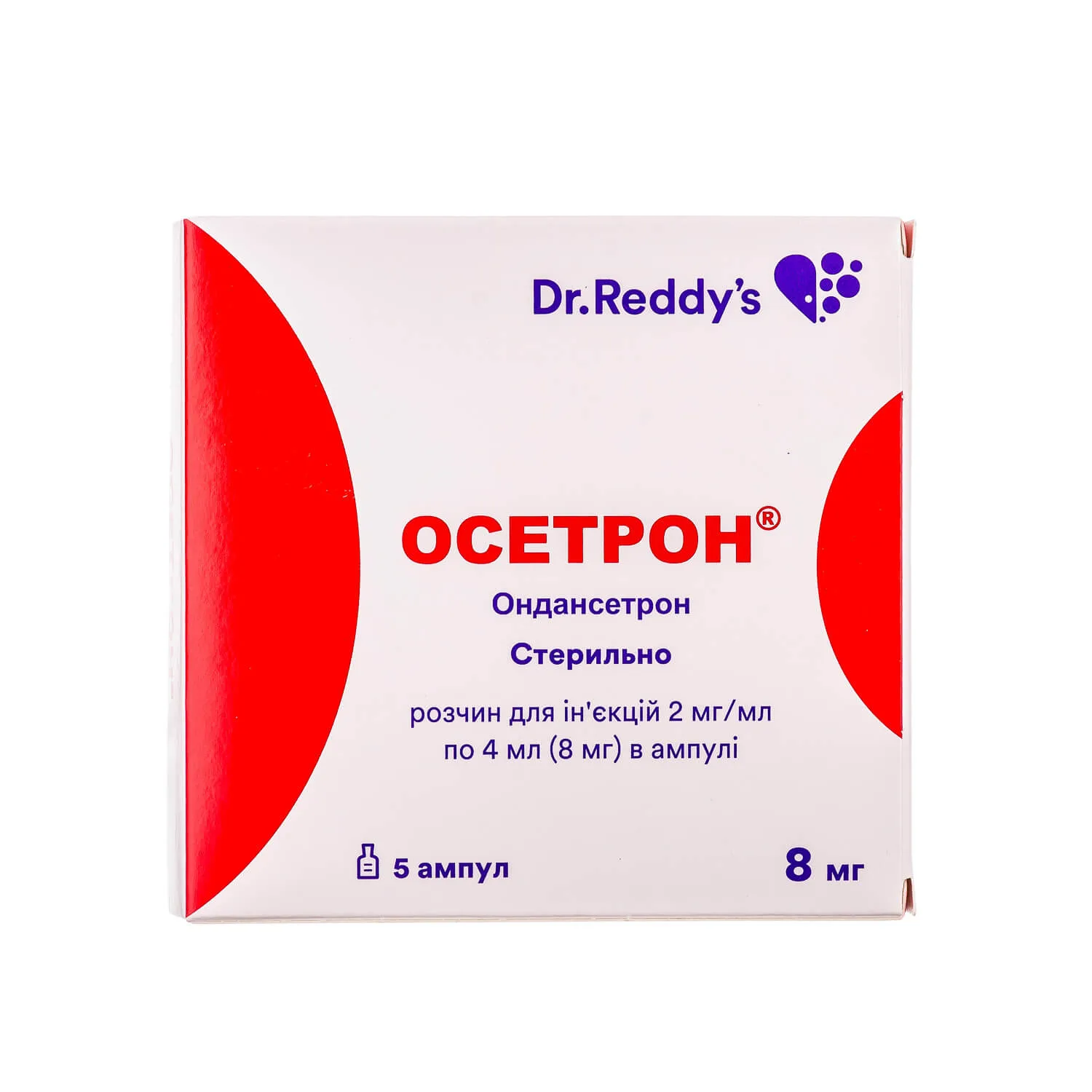
Osetron (Ondansetron) Ampoules 8 mg, 4 ml, №5
Ingredients
- Each ampoule contains 8 mg of ondansetron.
Dosage
- The recommended dosage is determined by a healthcare provider based on the individual’s condition.
- It is usually administered as a single dose before chemotherapy or radiation treatment.
Indications
- Osetron is indicated for the prevention of nausea and vomiting caused by chemotherapy and radiation therapy.
- It is also used to prevent and treat nausea and vomiting after surgery.
Contraindications
- Do not use Osetron if you are allergic to ondansetron or any of the other ingredients in the product.
- Inform your healthcare provider about any other medications you are taking to avoid potential drug interactions.
Directions
- Osetron is usually administered by injection into a vein or muscle by a healthcare professional.
- Follow all instructions provided by your healthcare provider.
Scientific Evidence
Ondansetron works by blocking serotonin, a natural substance that can trigger nausea and vomiting. Studies have shown its efficacy in preventing chemotherapy-induced nausea and vomiting.
Additional Information
- Osetron has been well-studied in clinical trials, demonstrating effectiveness in managing nausea and vomiting in cancer patients undergoing chemotherapy.
- It is considered a safe and reliable option for controlling these side effects.
Osetron (Ondansetron) Ampoules 8 mg, 4 ml, №5 provide a convenient and effective solution for individuals experiencing nausea and vomiting due to chemotherapy, radiation therapy, or surgery.