Description
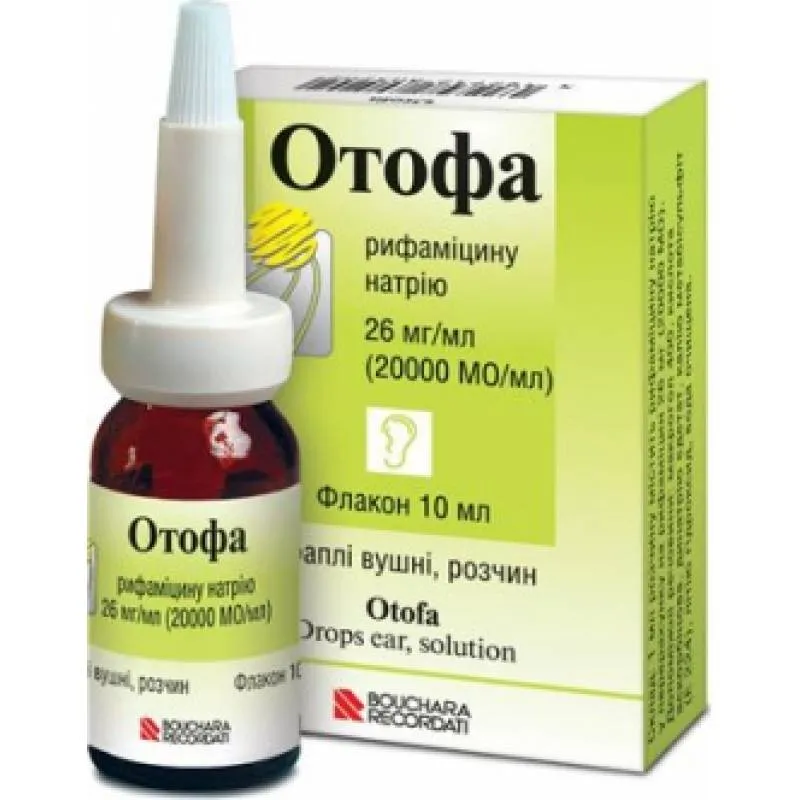
Otofa (Rifamycin) Ear Drops 26 mg, 10 ml
Ingredients
- Active ingredient: Rifamycin
Dosage
- Dosage: Instill 4 drops into the affected ear 3-4 times a day.
Indications
- Indications: Otofa ear drops are indicated for the treatment of bacterial ear infections.
Contraindications
- Contraindications: Do not use Otofa if you are allergic to rifamycin or any other ingredients in the product.
Directions
- Directions: Tilt your head to the side and instill the prescribed number of drops into the ear. Keep the head tilted for a few minutes to allow the drops to penetrate.
Scientific Evidence
Rifamycin, the active ingredient in Otofa ear drops, has demonstrated efficacy in treating bacterial ear infections. Studies have shown that rifamycin has potent antibacterial properties, targeting a wide range of bacteria commonly responsible for ear infections. Clinical trials have highlighted the effectiveness of rifamycin in reducing symptoms and eradicating bacterial pathogens in the ear canal.
Additional Information
Otofa ear drops provide targeted treatment directly to the site of infection, offering rapid relief from symptoms such as ear pain, discharge, and inflammation. The convenient dropper bottle ensures accurate dosing for effective treatment. It is important to complete the full course of treatment as prescribed by your healthcare provider to ensure the infection is fully cleared.