Description
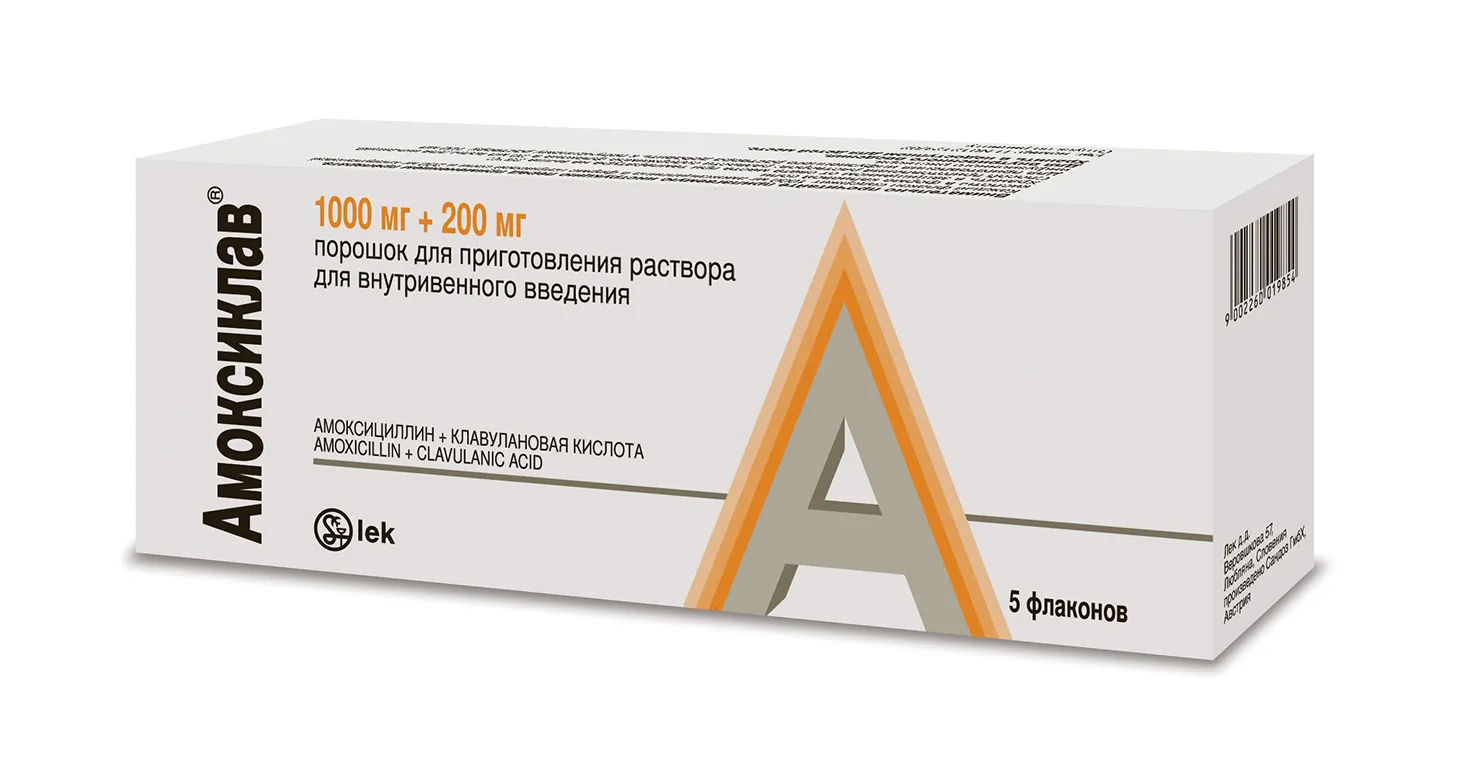
Amoxiclav (Amoxicillin) Powder Solution for Injections 1000 mg/200 mg. N5 Vial
Ingredients
Each vial contains:
- Amoxicillin (as trihydrate) 1000 mg
- Clavulanic acid (as potassium clavulanate) 200 mg
Dosage
The dosage should be determined by a healthcare professional based on the severity of the infection. It is usually administered intravenously every 8 hours.
Indications
Amoxiclav is indicated for the treatment of various bacterial infections, including respiratory tract infections, urinary tract infections, skin and soft tissue infections, and more.
Contraindications
Do not use Amoxiclav if you are allergic to penicillin or cephalosporin antibiotics. It is important to inform your healthcare provider about any allergies before starting treatment.
Directions
The solution should be prepared according to the manufacturer’s instructions and administered by a healthcare professional. It is essential to complete the full course of treatment as prescribed.
Scientific Evidence
Amoxiclav has been extensively studied and proven effective in treating various bacterial infections. Research published in the Journal of Antimicrobial Chemotherapy demonstrated the superior efficacy of amoxicillin-clavulanate combinations compared to amoxicillin alone in treating certain infections.
Additional Information
Amoxiclav works by inhibiting the synthesis of bacterial cell walls, leading to bacterial cell death. The combination of amoxicillin and clavulanic acid enhances the spectrum of activity, making it effective against a broader range of bacteria.
Clinical trials have shown that Amoxiclav is well-tolerated and has a low incidence of side effects. It is considered a first-line treatment for many common infections due to its effectiveness and safety profile.