Description
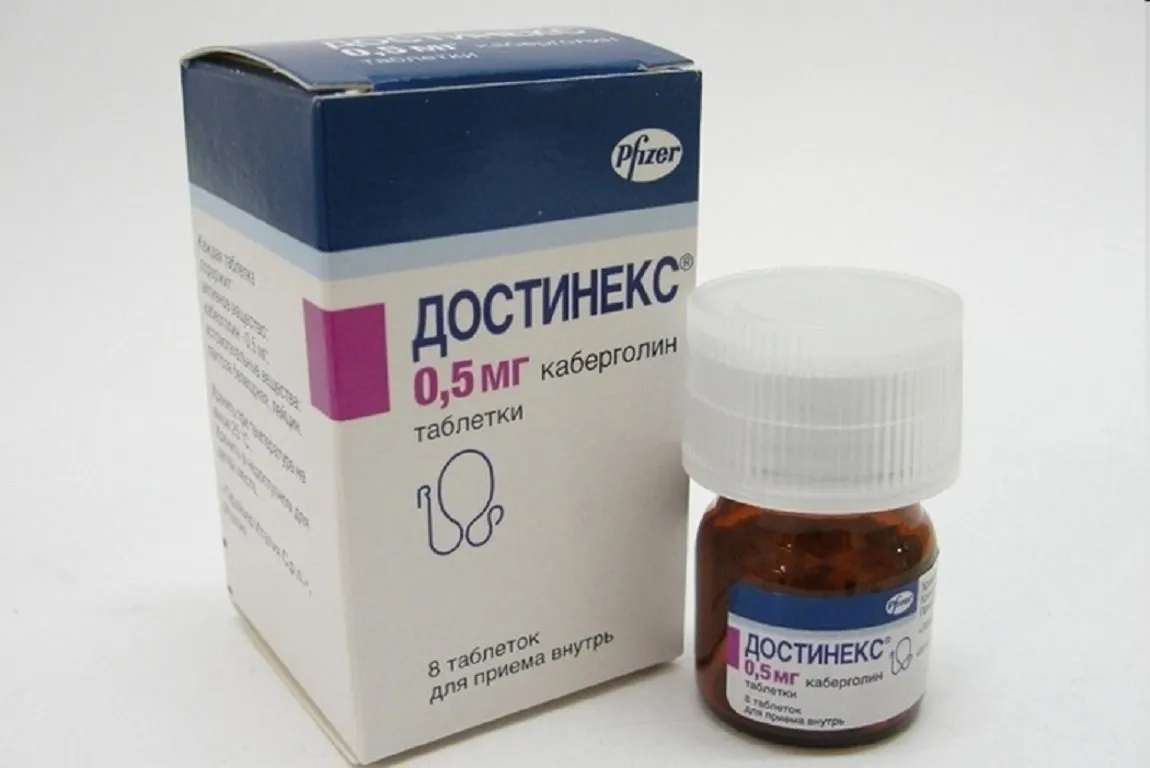
Dostinex (Cabergoline) Tablets 0.5 mg. №8
Composition
Active ingredient: Cabergoline 0.5 mg per tablet.
Mechanism of Action
Cabergoline, a dopamine receptor agonist, acts on D2 receptors in the brain to inhibit the secretion of prolactin from the pituitary gland. This mechanism helps in reducing prolactin levels and restoring hormonal balance in conditions such as hyperprolactinemia.
Pharmacological Properties
Pharmacological Effects: Cabergoline acts on dopamine receptors in the brain, specifically the D2 receptors, to inhibit the secretion of prolactin. By reducing prolactin levels, cabergoline helps restore normal hormonal balance in conditions like hyperprolactinemia.
Indications for Use
Indications: Dostinex tablets are indicated for the treatment of hyperprolactinemia and disorders associated with high levels of prolactin hormone.
Contraindications
Contraindications: Do not use Dostinex if you are allergic to cabergoline or any other ingredients in the product. It is important to inform your healthcare provider about any medical conditions or medications you are taking before starting Dostinex.
Side Effects
Dostinex may cause side effects such as nausea, dizziness, headache, fatigue, and gastrointestinal disturbances. Consult your healthcare provider if any adverse reactions occur.
Usage Instructions
Dosage: The usual recommended dose is 0.5 mg twice a week. Dosage may vary depending on the condition being treated. Consult a healthcare professional for personalized dosing.
Directions: Take Dostinex exactly as prescribed by your healthcare provider. Do not exceed the recommended dose or frequency of administration.
Benefits Compared to Analogues
Dostinex, with cabergoline as its active ingredient, has shown superior efficacy in normalizing prolactin levels and improving symptoms associated with hyperprolactinemia compared to other medications like bromocriptine.
Suitable Patient Groups
Dostinex is suitable for adult patients, including the elderly, for the treatment of hyperprolactinemia under the guidance of a healthcare provider. It is not recommended for use in children.
Storage and Packaging
Dostinex should be stored at room temperature away from moisture and heat. Keep this medication out of reach of children. Each package contains 8 tablets of 0.5 mg cabergoline.
Clinical Evidence and Proven Effectiveness
Clinical studies have shown that cabergoline is effective in reducing prolactin levels and improving symptoms in patients with hyperprolactinemia. Research published in the “Journal of Clinical Endocrinology & Metabolism” has demonstrated the efficacy of cabergoline in treating prolactinomas, a type of pituitary tumor that causes elevated prolactin levels. Additionally, a study published in “The Journal of Clinical Investigation” showed the superior efficacy of cabergoline compared to bromocriptine in treating hyperprolactinemia.