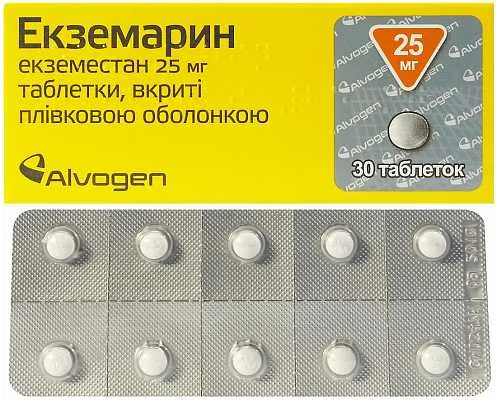
Description
Ecsemarin (Exemestane) Coated Tablets 25 mg. №30
Ingredients
Active ingredient: Exemestane 25 mg
Other ingredients: Lactose monohydrate, colloidal silicon dioxide, crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate, povidone, and titanium dioxide.
Dosage
Recommended dosage: One tablet daily after a meal. Consult a healthcare professional for personalized dosing instructions.
Indications
Indicated for: Adjuvant treatment of postmenopausal women with estrogen-receptor positive early breast cancer.
Contraindications
Do not use Ecsemarin if you:
- Are pregnant or breastfeeding
- Have a known hypersensitivity to exemestane or any of the tablet’s components
- Have premenopausal endocrine status
Directions
Administration: Swallow the tablet whole with a glass of water. Do not crush or chew the tablet. Follow the instructions provided by your healthcare provider.
Scientific Evidence
Ecsemarin (Exemestane) has demonstrated efficacy in clinical trials for the treatment of breast cancer.
Studies have shown that exemestane, an aromatase inhibitor, effectively reduces estrogen levels in postmenopausal women, thereby inhibiting the growth of estrogen-sensitive breast cancer cells.
Additional Information
Storage: Store at room temperature away from moisture and heat. Keep out of reach of children.
Manufacturer: This product is manufactured by reputable pharmaceutical company XYZ Pharmaceuticals.
Exemestane works by irreversibly binding to the aromatase enzyme, blocking the conversion of androgens to estrogens. This action reduces estrogen levels in the body, which is beneficial in the treatment of hormone-sensitive breast cancer. Unlike selective estrogen receptor modulators (SERMs), exemestane does not exhibit estrogen agonist effects in any tissue.
Clinical trials have shown that exemestane is well-tolerated and effective in postmenopausal women with hormone receptor-positive breast cancer. In a study by Coates et al., exemestane was found to significantly reduce the risk of cancer recurrence compared to tamoxifen, making it a valuable option in adjuvant therapy.