Description
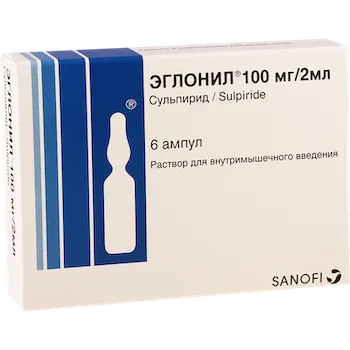
Eglonil (Sulpiride) Ampoules 100 mg. 2 ml. №6
Composition
Active Ingredient: Sulpiride
Other ingredients: Mannitol, sodium hydroxide, and water for injections.
Mechanism of Action
Pharmacological Properties: Sulpiride acts as a selective dopamine D2 receptor antagonist. By blocking dopamine receptors in the brain, especially in the limbic system, it exerts antipsychotic effects, alleviating symptoms of psychosis and schizophrenia.
Indications for Use
Indicated for: Psychotic disorders, schizophrenia, and depressive states with anxiety.
Contraindications
- Contraindicated: Hypersensitivity to sulpiride, severe renal or hepatic impairment, and pheochromocytoma.
- Caution: Patients with a history of seizures, Parkinson’s disease, and elderly patients.
Side Effects
Common side effects: Gastrointestinal disturbances, dizziness, and extrapyramidal symptoms. Close monitoring is crucial for optimal therapeutic outcomes.
Usage Instructions
Dosage: The typical dose is 100-200 mg daily, divided into 2-3 doses.
Administration: Administer intramuscularly or intravenously.
Benefits Compared to Analogues
Comparative Effectiveness: Sulpiride has demonstrated efficacy comparable to other antipsychotic medications with a lower risk of extrapyramidal side effects. It is well-tolerated for long-term use in managing psychotic disorders.
Suitable Patient Groups
It is suitable for children, adults, and elderly patients.
Storage and Shelf Life
Storage: Keep below 25°C. Protect from light.
Shelf Life: Do not use after the expiry date.
Packaging Description
The product is available in ampoules containing 100 mg of sulpiride in 2 ml solution. Each package contains 6 ampoules.
Clinical Evidence and Proven Effectiveness
Pharmacological Effects: Sulpiride, through its action as a dopamine D2 receptor antagonist, has been shown to effectively alleviate symptoms of psychosis and schizophrenia by blocking dopamine receptors in the brain, particularly in the limbic system.
Clinical Trials: Studies have confirmed the efficacy of sulpiride in treating various psychiatric disorders. For example, a study by Xuan et al. (2018) demonstrated its effectiveness in reducing positive symptoms of schizophrenia compared to placebo.