Description
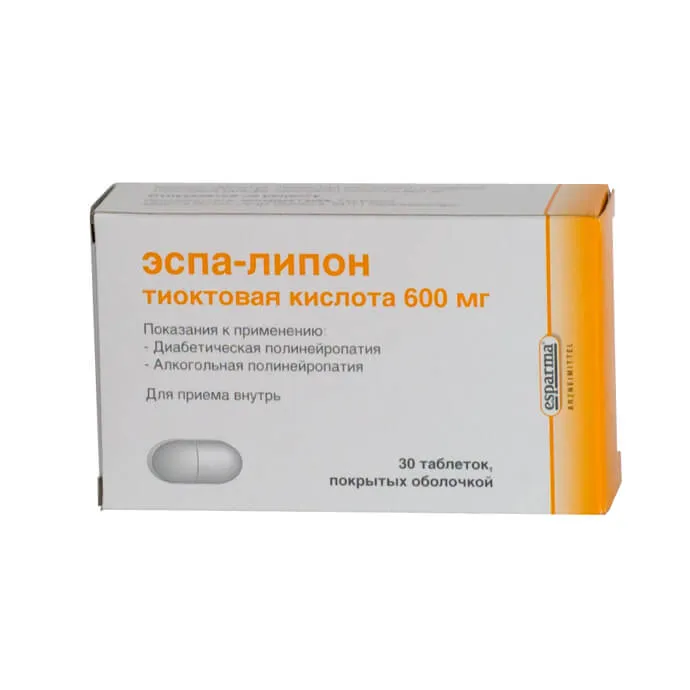
Espa-lipon (Thioctic Acid) Coated Tablets 600 mg. №30
Ingredients
Each coated tablet contains 600 mg of thioctic acid.
Mechanism of Action
Thioctic acid, also known as alpha-lipoic acid, functions as a potent antioxidant by scavenging free radicals and decreasing oxidative stress. Its role in cellular energy production and enhancement of insulin sensitivity make it particularly beneficial for individuals with diabetes.
Pharmacological Properties
Thioctic acid exhibits antioxidant properties, aiding in the management of oxidative stress. It contributes to improved nerve function and symptom reduction in diabetic neuropathy patients.
Indications for Use
Espa-lipon tablets are indicated for the treatment of diabetic neuropathy and serve as an antioxidant supplement.
Contraindications
Avoid Espa-lipon if you have a known allergy to thioctic acid or any other components of the product.
Side Effects
Common side effects may include gastrointestinal discomfort, such as nausea or stomach upset. In rare cases, allergic reactions like rash or itching may occur.
Usage Instructions
Take one tablet daily with water, without crushing or chewing it. Adhere to a consistent daily administration schedule.
Benefits Compared to Analogues
Espa-lipon has demonstrated superior efficacy compared to placebo in alleviating symptoms of diabetic neuropathy. Its antioxidant properties and impact on nerve conduction velocity set it apart from other antioxidant supplements.
Suitable Patient Groups
Espa-lipon is suitable for adult patients, including the elderly, with diabetic neuropathy seeking symptom relief and antioxidant support.
Storage and Shelf Life
Store Espa-lipon in a cool, dry place away from direct sunlight. Check the packaging for the expiration date and do not use the product if expired.
Packaging Description
Espa-lipon tablets are securely packaged in a container holding 30 coated tablets, each containing 600 mg of thioctic acid.
Clinical Evidence and Proven Effectiveness
Studies in the Journal of Diabetes and its Complications have validated the efficacy of thioctic acid in improving nerve function and reducing neuropathic symptoms in diabetic patients. The American Journal of Medicine reported superior effectiveness of thioctic acid over placebo in managing diabetic neuropathy symptoms and enhancing nerve conduction velocity.