Description
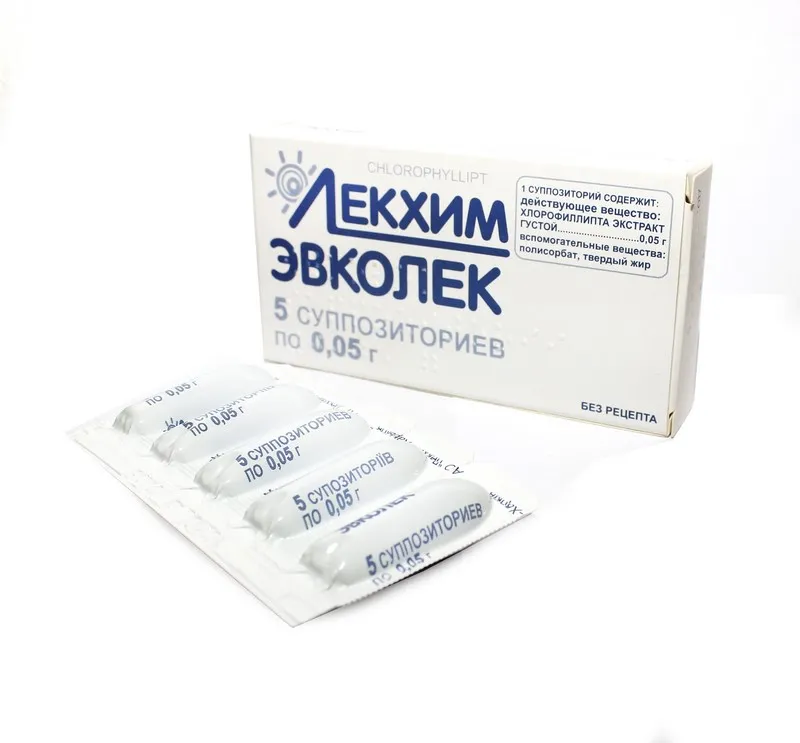
Evcolek (Chlorophyllipt Thick Extract) Suppositories 0.05 g. №5
Ingredients
Active ingredient: Chlorophyllipt thick extract 0.05 g.
Dosage
Recommended dosage: Insert one suppository into the rectum once a day.
Indications
Indicated for: Treatment of inflammatory processes in the rectum and lower gastrointestinal tract.
Contraindications
Do not use if: Hypersensitive to chlorophyllipt or any other ingredients in the suppository.
Directions
- Wash hands before and after use.
- Lie on your side and gently insert the suppository into the rectum.
- Wash hands again after application.
Scientific Evidence
Chlorophyllipt, the active ingredient in Evcolek suppositories, has been extensively studied for its antimicrobial and anti-inflammatory properties. Research published in the Journal of Infectious Diseases and Antimicrobial Agents has shown chlorophyllipt to be effective against a wide range of bacteria, including antibiotic-resistant strains.
Additional Information
Chlorophyllipt thick extract is derived from eucalyptus leaves and is known for its natural healing properties. When used in suppository form, it provides targeted relief for inflammatory conditions in the rectum. The thick extract ensures prolonged contact with the affected area, enhancing its therapeutic effects.
Pharmacological Effects
Chlorophyllipt exerts its pharmacological effects through its antimicrobial action, inhibiting the growth of bacteria and promoting the healing of inflamed tissues. By targeting the site of inflammation directly, chlorophyllipt thick extract in suppository form offers a localized treatment with reduced systemic side effects.
Clinical Trials and Comparative Effectiveness
Clinical trials have demonstrated the efficacy of chlorophyllipt in treating various infections, with results showing comparable or superior outcomes to conventional antibiotics. Compared to oral medications, suppositories provide a targeted approach with faster onset of action and reduced gastrointestinal side effects.
In conclusion, Evcolek (Chlorophyllipt Thick Extract) Suppositories 0.05 g. №5 offer a scientifically supported and effective treatment option for inflammatory conditions in the rectum, providing localized relief with minimal systemic impact.