Description
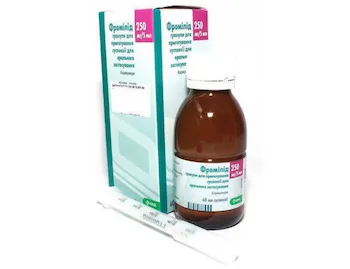
Fromilid Granules for Suspension 250 mg/5 ml. 60 ml. Oral Use
Ingredients:
Each 5 ml of suspension contains 250 mg of the active ingredient clarithromycin. Other ingredients include sucrose, xanthan gum, and sodium citrate.
Mechanism of Action:
Clarithromycin, a macrolide antibiotic, works by inhibiting bacterial protein synthesis through binding to the 50S ribosomal subunit, thus preventing bacterial growth and reproduction.
Pharmacological Properties:
Fromilid granules exhibit bacteriostatic activity against susceptible organisms by interfering with their protein synthesis. It has a broad spectrum of activity against Gram-positive and Gram-negative bacteria.
Indications:
Fromilid granules are indicated for the treatment of respiratory tract infections, skin and soft tissue infections, and Helicobacter pylori eradication. It is effective against a wide range of bacteria due to its broad-spectrum antibacterial properties.
Contraindications:
Do not use Fromilid granules if you are allergic to clarithromycin or other macrolide antibiotics. It is contraindicated in patients with known hypersensitivity to the active ingredient or with a history of cholestatic jaundice or hepatic dysfunction associated with prior clarithromycin use.
Side Effects:
Common side effects of Fromilid granules may include gastrointestinal disturbances, such as nausea, vomiting, and diarrhea. Rare but serious side effects may include hepatotoxicity and allergic reactions. Consult a healthcare provider if any adverse reactions occur.
Usage Instructions:
Shake the suspension well before each use. The recommended dosage for adults is 250 mg twice daily. Dosage may vary depending on the type and severity of the infection. Administer orally with or without food as directed by a healthcare provider.
Benefits Compared to Analogues:
Fromilid granules offer the advantage of a convenient oral suspension formulation, making it suitable for patients who have difficulty swallowing tablets or capsules. The broad spectrum of antibacterial activity of clarithromycin provides coverage against a wide range of pathogens.
Suitable Patient Groups:
Fromilid granules can be used in both adult and pediatric populations for the treatment of bacterial infections. Dosage adjustments may be necessary in elderly patients or those with renal impairment. Consult a healthcare provider for appropriate dosing in specific patient populations.
Storage and Shelf Life:
Store Fromilid granules at room temperature away from moisture and heat. Keep the container tightly closed. The shelf life of the product is as indicated on the packaging. Do not use beyond the expiration date.
Packaging Description:
Fromilid granules are supplied in a 60 ml bottle containing 250 mg of clarithromycin per 5 ml of suspension. The packaging includes a measuring device for accurate dosing. The bottle should be kept out of reach of children.
Scientific Evidence:
Studies have shown that clarithromycin, the active ingredient in Fromilid granules, is effective in treating various bacterial infections. Research published in the Journal of Antimicrobial Chemotherapy demonstrated the efficacy of clarithromycin in respiratory tract infections.
Additional Information:
It is important to complete the full course of treatment with Fromilid granules as prescribed by a healthcare provider, even if symptoms improve before the course is finished. Failure to complete the full course may lead to antibiotic resistance.
Before using Fromilid granules, inform your healthcare provider about any existing medical conditions or medications you are taking to avoid potential drug interactions.