Description
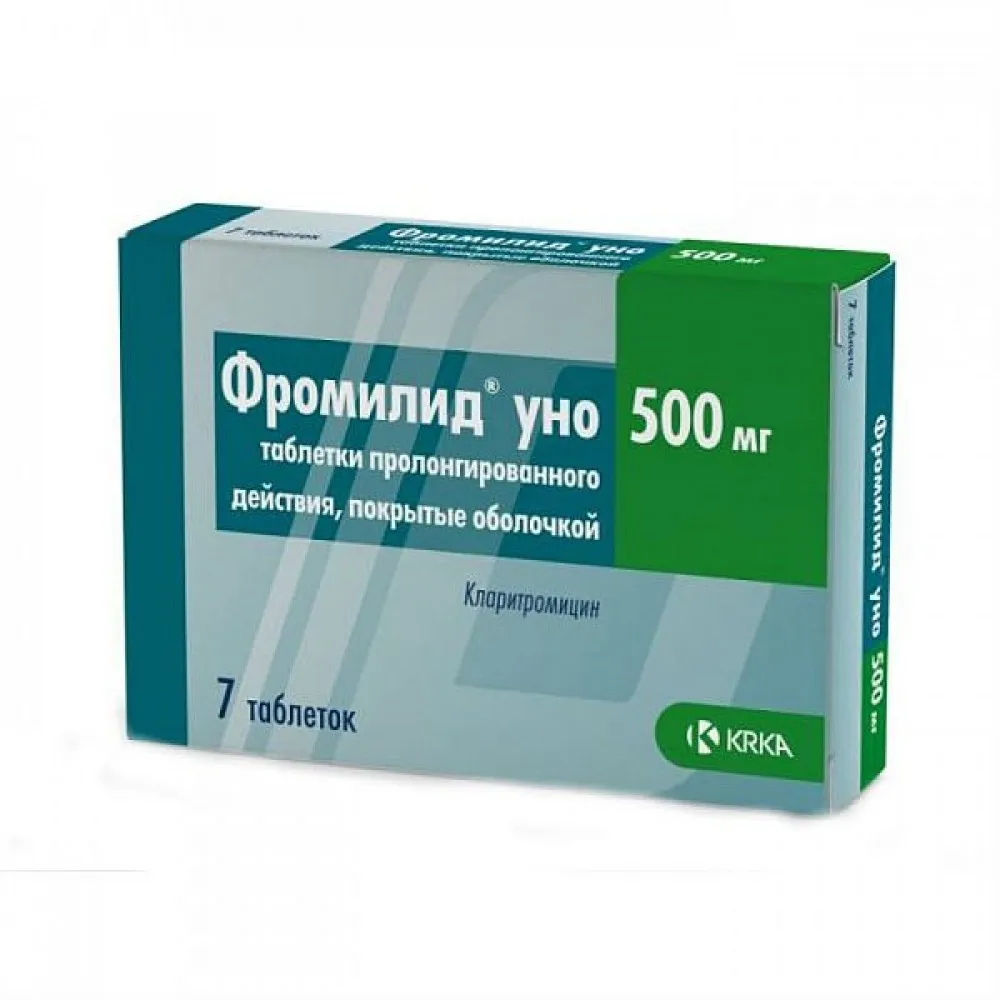
Fromilid UNO Tablets with Modified Release 500 mg. №7
Ingredients:
Each tablet contains 500 mg of modified release clarithromycin.
Mechanism of Action:
The pharmacological action of clarithromycin involves inhibiting bacterial protein synthesis, leading to the eradication of susceptible bacteria.
Indications for Use:
Fromilid UNO tablets are indicated for the treatment of bacterial infections. They are commonly prescribed for respiratory tract infections, skin and soft tissue infections, and Helicobacter pylori eradication in peptic ulcer disease.
Contraindications:
Do not take Fromilid UNO if you are allergic to clarithromycin or other macrolide antibiotics. It is important to inform your doctor about any allergies or medical conditions before starting this medication.
Side Effects:
Common side effects may include gastrointestinal disturbances, such as nausea and diarrhea. Rare but serious side effects may include allergic reactions like rash or difficulty breathing.
Usage Instructions:
Swallow the tablet whole with a glass of water, do not crush or chew it. The recommended dosage is one tablet once daily. It is best to take it at the same time each day to maintain a consistent level of the drug in your body.
Benefits Compared to Analogues:
Fromilid UNO tablets with modified release 500 mg provide a convenient once-daily dosing option for patients requiring treatment for bacterial infections. The modified release formulation ensures a steady concentration of the drug in the body, enhancing its effectiveness.
Suitable Patient Groups:
This medication is suitable for adults and adolescents. Dosage adjustments may be necessary for elderly patients or those with impaired renal function. It is not recommended for use in children under certain age groups.
Storage and Shelf Life:
Store the tablets in a cool, dry place away from direct sunlight. Keep them out of reach of children. Check the expiry date on the packaging and do not use the tablets if expired.
Packaging Description:
Fromilid UNO tablets are typically packaged in blister packs containing 7 tablets. The packaging should be intact and sealed before use.
Clinical Evidence and Proven Effectiveness:
Studies have shown the efficacy of clarithromycin in treating various bacterial infections. Research published in the International Journal of Antimicrobial Agents demonstrated the effectiveness of clarithromycin in the treatment of community-acquired pneumonia.