Description
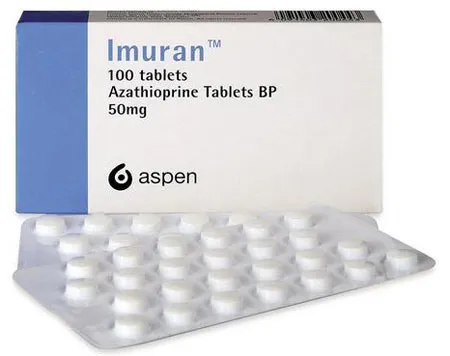
Imuran Coated Tablets 50 mg. №100
Composition
Active ingredient: Azathioprine.
Other ingredients: Lactose, maize starch, povidone, and magnesium stearate.
Mechanism of Action
Imuran interferes with DNA synthesis in immune cells, reducing their activity. This action helps prevent organ rejection and suppress abnormal immune responses in autoimmune diseases.
Pharmacological Properties
Imuran is an immunosuppressive agent that inhibits DNA synthesis in rapidly dividing cells, including those of the immune system. It modulates the immune response to prevent rejection of transplanted organs and manage autoimmune conditions.
Indications for Use
Imuran tablets are indicated for:
- Prevention of rejection in organ transplantation.
- Treatment of rheumatoid arthritis.
- Management of severe psoriasis.
Contraindications
Do not use Imuran tablets if:
- You are allergic to azathioprine or any other ingredients.
- You have a history of bone marrow suppression.
- You are pregnant or breastfeeding.
Side Effects
Common side effects of Imuran may include nausea, vomiting, diarrhea, and increased risk of infections. Serious side effects may include bone marrow suppression, liver toxicity, and increased risk of certain cancers.
Usage Instructions
Dosage: The usual dose ranges from 1 to 3 mg per kg of body weight per day, taken as a single dose or divided into two doses.
Directions for use: Swallow the tablets whole with a full glass of water. Do not crush or chew the tablets.
Benefits Compared to Analogues
Imuran offers the advantage of effectively preventing organ rejection in transplant recipients while also providing therapeutic benefits in autoimmune diseases like rheumatoid arthritis and severe psoriasis. Its immunosuppressive properties make it a valuable option for managing these conditions.
Suitable Patient Groups
Imuran can be used in various patient groups, including adults, children, and the elderly. However, caution is advised in pregnant or breastfeeding individuals and those with a history of bone marrow suppression.
Storage and Shelf Life
Storage: Store Imuran tablets at room temperature away from moisture and heat.
Shelf Life: Check the packaging for the expiry date and do not use expired tablets.
Packaging Description
Imuran tablets are typically packaged in a blister pack or bottle, containing 100 coated tablets of 50 mg each.
Clinical Evidence and Proven Effectiveness
Imuran has been supported by clinical studies demonstrating its efficacy in maintaining long-term graft survival in organ transplant recipients. Additionally, research has shown that combining Imuran with other immunosuppressive agents can enhance treatment outcomes in autoimmune conditions.