Description
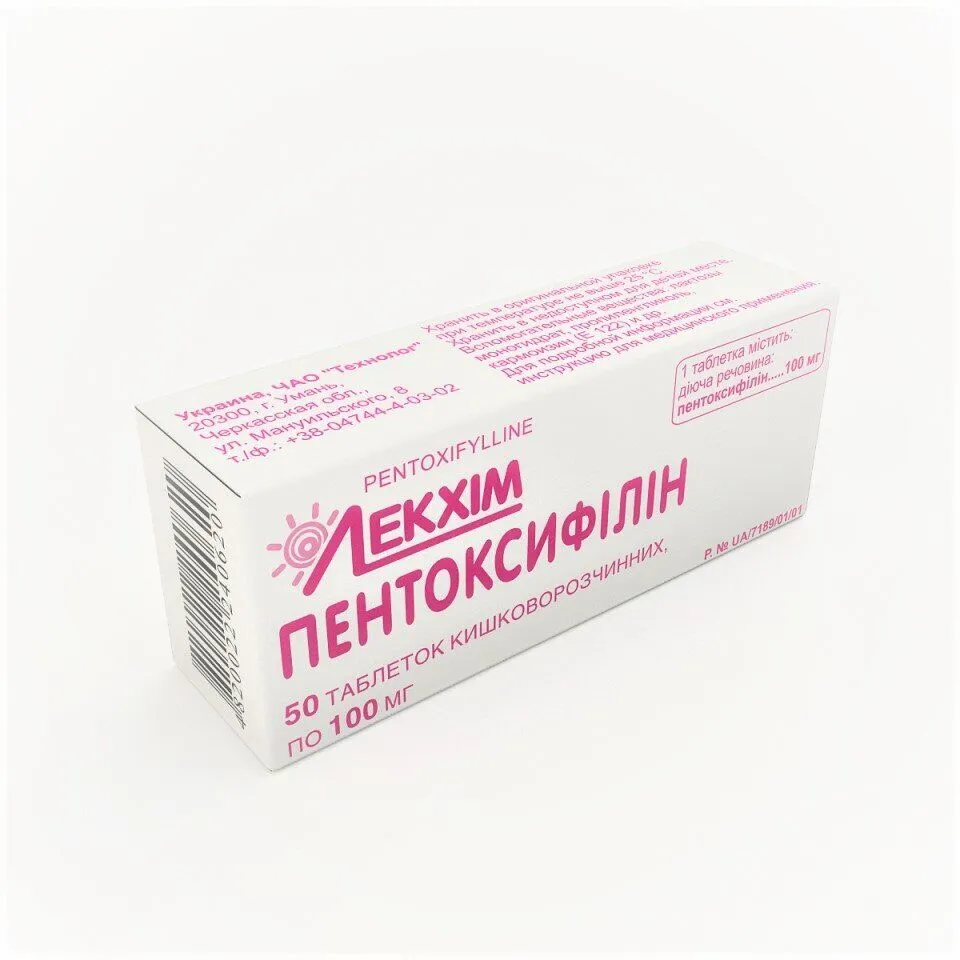
Pentoxifillin (Pentoxifylline) Enteric Tablets 0.1 g №50
Ingredients:
Each enteric tablet contains 0.1 g of Pentoxifylline.
Dosage:
The usual dosage is one tablet three times a day with meals. Dosage may vary based on individual patient needs and medical condition.
Indications:
Pentoxifylline is indicated for the treatment of peripheral vascular diseases. It helps improve blood flow by decreasing its viscosity, which in turn enhances oxygen delivery to tissues.
Contraindications:
Do not use Pentoxifylline if you have had recent cerebral and/or retinal hemorrhage. Consult a healthcare provider before use if you have a history of bleeding disorders.
Directions:
Swallow the tablets whole with a full glass of water. Do not crush or chew the tablets. Follow the dosage instructions provided by your healthcare provider.
Scientific Evidence:
Pentoxifylline, the active ingredient in Pentoxifillin enteric tablets, has been extensively studied for its efficacy in improving peripheral blood flow and microcirculation. Research published in the Journal of Vascular Surgery demonstrated that Pentoxifylline significantly increased walking distance in patients with intermittent claudication, a common symptom of peripheral artery disease.
Additional Information:
Pentoxifylline exerts its pharmacological effects by inhibiting phosphodiesterase and adenosine uptake, leading to vasodilation and improved erythrocyte flexibility. Clinical trials have shown that Pentoxifylline not only improves symptoms of peripheral vascular diseases but also enhances tissue oxygenation and wound healing. It is considered a valuable adjunct in the management of peripheral artery disease.