Description
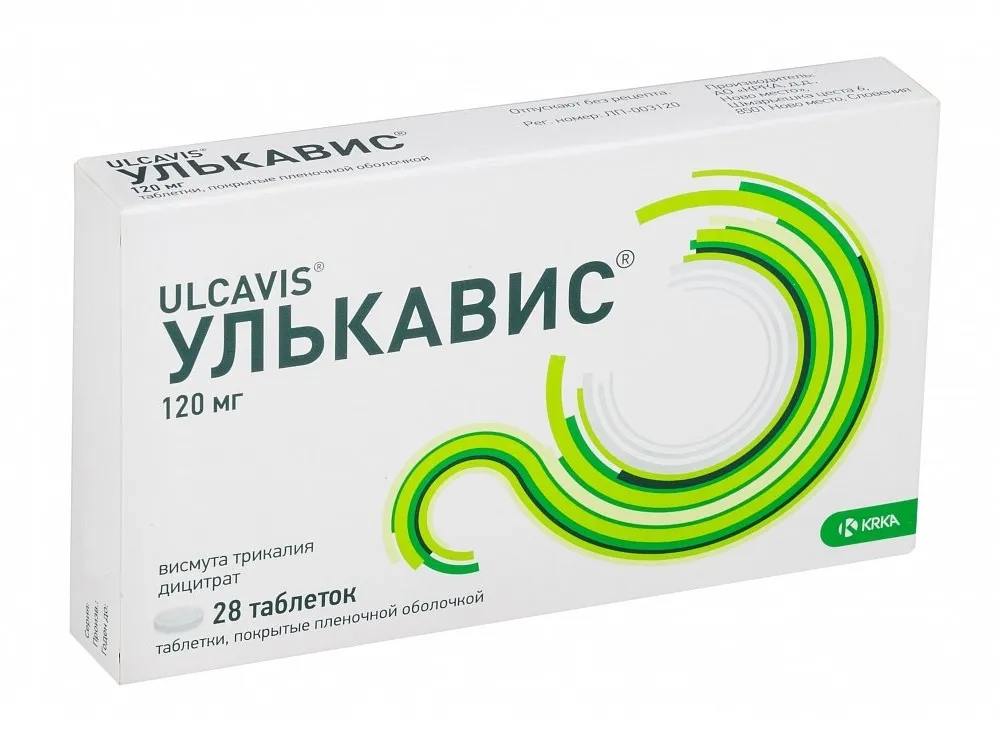
Ulcavis (Bismuth Subcitrate) Coated Tablets 120 mg. №28
Ingredients:
Each coated tablet contains 120 mg of bismuth subcitrate.
Dosage:
The recommended dosage is one tablet to be taken orally three times a day, preferably after meals.
Indications:
- Ulcavis tablets are indicated for the treatment of peptic ulcers.
- They are also used in the management of Helicobacter pylori infection.
Contraindications:
Do not use Ulcavis tablets if you are allergic to bismuth subcitrate or any other ingredients in the formulation.
Directions:
Swallow the tablet whole with water. Do not crush or chew the tablet.
Scientific Evidence:
Bismuth subcitrate, the active ingredient in Ulcavis tablets, inhibits the growth of Helicobacter pylori bacteria, aiding in ulcer healing and prevention of recurrence.
Additional Information:
Ulcavis tablets are well-tolerated with minimal side effects, making them a preferred choice for peptic ulcer treatment. Proper adherence to the prescribed dosage and treatment duration is crucial for optimal results.
Combination therapy involving bismuth subcitrate has shown promise in reducing antibiotic resistance in Helicobacter pylori eradication regimens, emphasizing its role in gastrointestinal disorder management.