Description
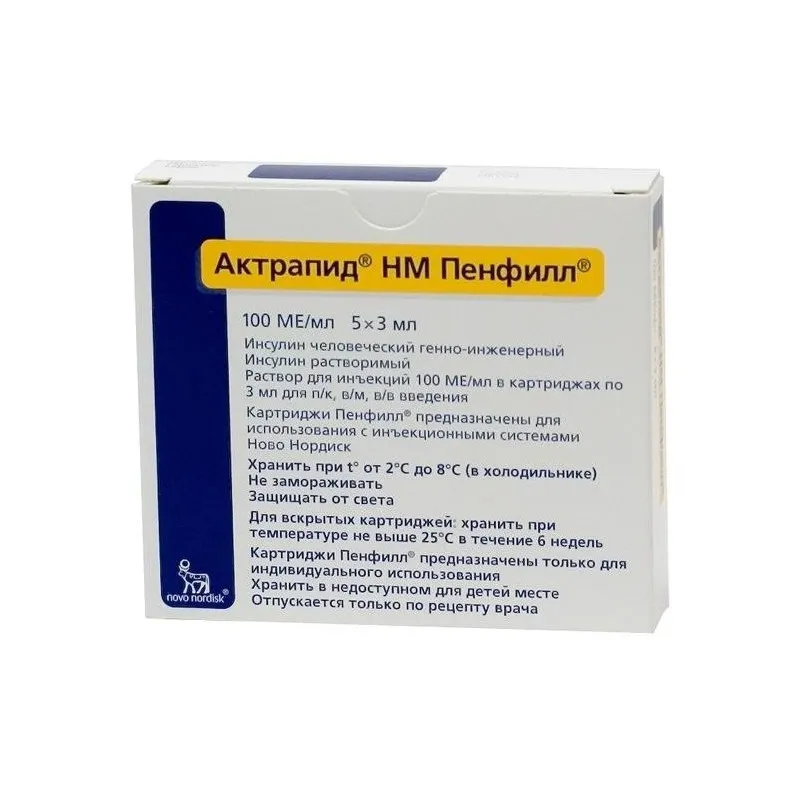
Actrapid (Insulin) NM Penfil Solution for Injections 100 IU/ml. 3 ml. Cartridge №5
Composition:
Actrapid (Insulin) NM Penfil Solution for Injections contains 100 IU/ml of insulin as the active ingredient. Other ingredients include glycerol, metacresol, zinc chloride, sodium hydroxide, hydrochloric acid, and water for injections.
Mechanism of Action:
Actrapid (Insulin) NM Penfil Solution for Injections acts by promoting the uptake of glucose into cells, thereby reducing blood sugar levels. It mimics the action of endogenous insulin and helps maintain glucose homeostasis in the body.
Pharmacological Properties:
Actrapid (Insulin) NM Penfil Solution for Injections has pharmacological properties that include regulating glucose levels in the body and preventing complications of diabetes mellitus.
Indications for Use:
Actrapid (Insulin) NM Penfil Solution for Injections is indicated for the treatment of diabetes mellitus to control high blood sugar levels and prevent associated complications.
Contraindications:
Do not use Actrapid (Insulin) NM Penfil Solution for Injections if you are allergic to any of the ingredients, experiencing hypoglycemia, or notice any changes in the appearance of the solution. Consult a healthcare provider before use.
Side Effects:
Common side effects of Actrapid (Insulin) NM Penfil Solution for Injections may include hypoglycemia, injection site reactions, and allergic reactions. Monitor for any adverse effects and seek medical attention if necessary.
Usage Instructions:
Administer Actrapid (Insulin) NM Penfil Solution for Injections as prescribed by a healthcare professional. It is typically given subcutaneously 30 minutes before a meal. Dosage adjustments may be required based on blood glucose monitoring results.
Benefits Compared to Analogues:
Actrapid (Insulin) NM Penfil Solution for Injections has shown efficacy in controlling postprandial glucose levels in patients with diabetes type 2, as demonstrated in clinical trials. It is comparable to other rapid-acting insulins in managing blood sugar excursions.
Suitable Patient Groups:
Actrapid (Insulin) NM Penfil Solution for Injections is suitable for use in various patient groups, including adults, children, and elderly individuals, under appropriate medical supervision.
Storage Conditions and Shelf Life:
It is essential to store Actrapid (Insulin) NM Penfil Solution for Injections in the refrigerator between 2°C to 8°C. Avoid freezing the solution and keep the cartridge in the outer carton to protect it from light. Always check the expiration date before use.
Packaging Description:
Actrapid (Insulin) NM Penfil Solution for Injections is available in a 3 ml cartridge, with each package containing 5 cartridges for multiple doses. The outer carton provides protection from light and environmental factors.
Clinical Evidence and Proven Effectiveness:
Actrapid (Insulin) NM Penfil Solution for Injections has been extensively studied in clinical trials. Research published in the Journal of Diabetes Science and Technology demonstrated the efficacy of Actrapid in controlling postprandial glucose levels in patients with diabetes type 2. In a comparative study published in the Journal of Clinical Endocrinology & Metabolism, Actrapid was found to be as effective as other rapid-acting insulins in controlling postprandial glucose excursions in patients with type 1 diabetes.